












The "Atlas of TB Innovations" is catalogue of all innovations in the fight against Tuberculosis (TB).
This section showcases a select few innovations that are driving global progress in the fight against TB and beyond.
Truelyse™, a rapid sample preparation system by Molbio Diagnostics Limited, addresses this most persistent barrier in TB diagnosis. Designed to make TB testing faster, simpler, and more patient-friendly, especially for the key population, Truelyse uses oral swab-based testing to detect TB.
While sputum remains the standard specimen for pulmonary TB, reliance on sputum-based diagnosis often excludes key populations—children, older adults, people living with HIV, and immunocompromised patients—who are unable to produce an adequate sputum sample. As a result, nearly one in four people with TB—and a significantly higher proportion among children and PLHIV—risk being delayed or missed within the care cascade.

How it works:
Unlike conventional workflows that depend on complex extraction reagents and time-intensive processing, Truelyse uses high-frequency mechanical agitation to break open bacterial cells in about 90 seconds. This process efficiently releases nucleic acids that can be directly amplified on Truenat MTB Ultima, enabling a seamless and accelerated path from sample collection to TB detection, cutting down the sample-to-result time in half.
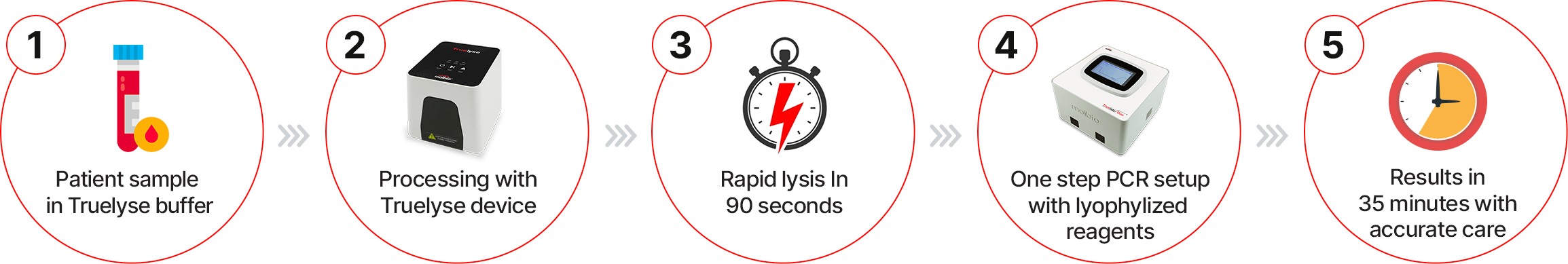
Truelyse at a Glance:
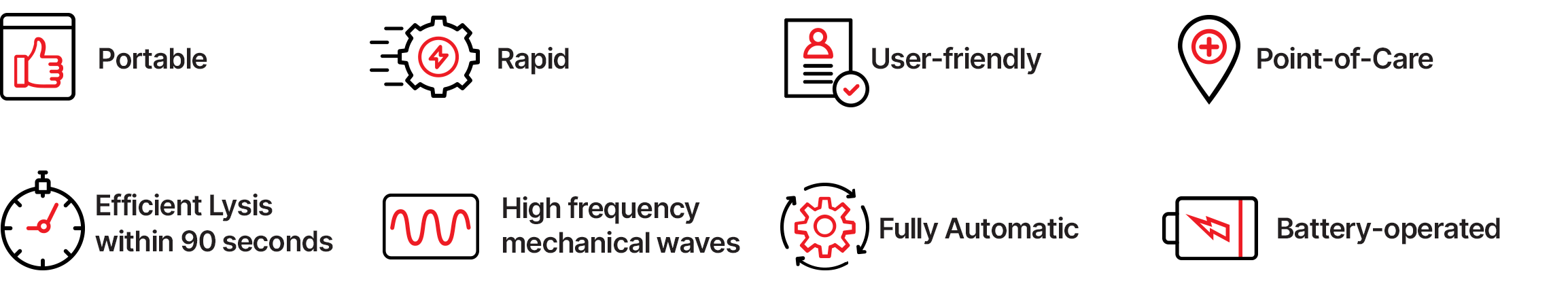
Oral swab-based TB testing with Truelyse and Truenat MTB Ultima offers significant advantages:
By expanding sample options and simplifying molecular workflows, Truelyse™ strengthens TB diagnosis in settings where traditional sputum-based testing often becomes a bottleneck. It is particularly valuable in pediatric TB and HIV–TB co-infection contexts and supports community screening and field-based diagnosis, helping bring molecular TB testing closer to where patients first seek care.
Conclusion:
Truelyse™ enables a more inclusive, decentralized, and practical approach to TB diagnosis at the point of care.
Autonomous Mobile AI Integrated UV‑C Disinfection Robot refers to mobile robots developed by Haystack Robotics, aimed at performing disinfection tasks in indoor environments using UV‑C light, with AI and robotics for automation. It comes under the category of filtration & disinfection innovations in India. These robots are meant to reduce infectious pathogens (viruses, bacteria, fungi, and spores) on surfaces and in air, without relying on chemical disinfectants. They are designed for hospitals, public spaces, offices, etc.

Key Features & Capabilities
Here are the main technical/functional features of the Haystack UVC robots:
Feature | Description |
Autonomous Navigation | The robot is mobile and moves through rooms and corridors without needing manual steering. It does not require pre-mapping of rooms in many cases. |
AI Integration | The robot uses AI for tasks such as object recognition, detecting “high-touch” surfaces (doorknobs, light switches), and adjusting its path or disinfection dwell time over those surfaces. |
Safety Features | Human detection: when a person is detected in the room, the robot enters safe mode (pauses or dims UV light) to prevent exposure. No harsh chemicals or residues since disinfection is via UV‑C light. |
Verifiable Disinfection Reports | The system can generate coverage reports, maps showing which areas have been disinfected, timestamps, total time, etc. This allows for auditing and tracking. |
Efficiency & Time | Disinfection times are relatively short: e.g., an average room (size ~15×15 feet) in ~10 minutes. |
Runtime/Battery | The robot has a high capacity. Battery capable of several hours of operation (an 8-hour runtime is cited for some models). |
Ease of Use | Designed to be user-friendly: learn to operate in ~15 minutes, simple interface, pushbutton operation, and possibly “Follow Me” mode. |
Over-the-Air Software Updates | The robots support remote software updates to improve features or fix bugs without requiring manual hardware intervention. |
Mobility while Disinfecting | Unlike many UVC systems, which are static (the lamp stays in one place), these robots move around during disinfection, helping cover shadowed areas. |
Use Cases & Advantages:
qXR is an AI-powered chest X-ray interpretation tool developed by Qure.ai. Trained on over 10 million scans, it provides pre-read assistance in less than a minute, detecting abnormalities across the lungs, heart, pleura, mediastinum, bones, and diaphragm. qXR has been deployed in over 100 countries across 4700+ sites, processing more than 35 million scans. It has achieved a 40% reduction in turnaround time for reporting by classifying normal X-rays with a 99% negative predictive value. The tool supports multilingual interfaces and can be deployed on the cloud or on-premise, making it adaptable to various healthcare settings globally.
Quantiplus® MTB Fast Detection Kit
Developed by Huwel Lifesciences, the Quantiplus® MTB Fast Detection Kit is a rapid, extraction-free, open RT-PCR solution designed to overcome key diagnostic barriers. It delivers confirmatory TB results in under 40 minutes using standard PCR machines, eliminating the need for proprietary equipment and complex workflows. This makes Quantiplus® both affordable and scalable for decentralized TB testing.

Despite advances in molecular diagnostics, tuberculosis detection in India continues to face major barriers. High costs, proprietary closed systems, infrastructure demands, and limited geographic reach prevent widespread adoption.
Limitations of Existing Methods
Key Features of the Quantiplus® MTB Fast Detection Kit:
Proven Performance and Real-World Validation
Laboratory and ICMR-led evaluations have demonstrated strong diagnostic performance. The field study demonstrated 85.03% sensitivity, 91.64% specificity, and 90.08% overall diagnostic accuracy. Field feasibility studies in decentralized settings confirmed reliable performance, operational simplicity, and suitability for high-volume testing. A health technology assessment is underway to further support its potential for scale-up under the National Tuberculosis Elimination Programme (NTEP).
Enabling Access, Accelerating Impact
Priced at approximately ₹150 per test, Quantiplus® brings laboratory-grade molecular accuracy to the point of care. By enabling rapid, reliable, and affordable TB diagnosis in underserved settings, it empowers healthcare systems to detect cases earlier, initiate treatment faster, and reduce transmission—driving meaningful progress toward TB elimination.
Implementation Progress
Significant advancements include completed Quantiplus Nikshay interoperability (ICMR-approved for imminent rollout). State-level evaluations: Tamil Nadu (three district sites) and Uttar Pradesh (integration with ~85 COVID-era RT-PCR instruments). The Swaminathan Foundation supports uptake in Tamil Nadu and Odisha.
Key Initiatives