










Atlas of TB Innovations: Mapping the Future
The "Atlas of TB Innovations" is catalogue of all innovations in the fight against Tuberculosis (TB).
Featured Innovations
This section showcases a select few innovations that are driving global progress in the fight against TB and beyond.
ProDigi Health Platform for TB screening with PRORAD ATLAS ULTRAPORTABLE Digital X-Ray system
The PRORAD Atlas Ultraportable X‑Ray System is a cutting-edge, backpack-style radiography solution designed for definitive imaging anytime, anywhere—from remote health camps to bedside diagnostics.

Key Highlights
- Ultra-Lightweight & Portable: Weighing approximately 2.80 kg (including battery), this featherweight unit offers exceptional mobility.
- Rapid Setup — “Aim and Expose”: Engineered for efficiency with intuitive ergonomics for field use and quick patient imaging.
- Smart, User-Friendly Control Interface: Features a digital or touchscreen display to set exposure parameters with ease.
- Extended Battery Life: Equipped with a rechargeable battery (11.1 VDC), it ensures prolonged field usage.
- Ultra-Low Radiation Dose: Delivers exceptional image quality while maintaining a low radiation exposure profile, thanks to excellent MTF and DQE performance.
- Seamless DICOM Integration: Designed for streamlined image handling and connectivity with health IT systems.
Technical Specifications of PROGNOSYS MEDICAL SYSTEMS (PRORAD)
PROGNOSYS MEDICAL SYSTEMS (PRORAD) | |||
Parameter | PRORAD ATLAS Ultraportable | PRORAD ATLAS Ultraportable Plus | PRORAD ATLAS ULTIMA Ultraportable |
Weight (kg) | 2.80 kg | 3.0 kg | 4.5 kg |
External Dimensions (mm) | 220mm x 170mm x 135mm | 220mm x 170mm x 135mm | 324mm x 180mm x 182mm |
Input power | 11.1 V (Lithium-ion Polymer) | 22.2 V (Lithium-ion Polymer) | 22.2 V (Lithium-ion Polymer) |
Radiation Exposure Time (seconds) | 0.01 – 1.3 sec | 0.01 – 2.0 sec | 0.01 – 2.0 sec |
Tube Current (mA) | 2 mA | 6 mA | 10 mA |
kV Peak (Penetration Power) | 70 kV | 70 kV | 90 kV |
Collimator | Cone Collimator with Cross hair Laser | Cone Collimator with Cross hair Laser | Manual Multi leaf collimator with LED light and cross hair laser |
Focal Point Size (mm) | 0.4 mm | 0.4 mm | 0.8 mm |
Anode Characteristics | Stationary Anode. Heat storage capacity of 4500 J (6,435 HU) | Stationary Anode. Heat storage capacity of 4500 J (6,435 HU) | Stationary Anode. Heat storage capacity of 8000 J (11,440 HU) |
Output Power (W) | 140 W | 420 W | 500 W |
Regulatory Approval & Intended Use
AERB, CDSCO, BIS, BAPTEN (Indonesia), FDA Philippines, US FDA Approved
Why It Stands Out
- Unmatched Mobility: Its compact size and light weight enable healthcare professionals to deliver imaging services virtually anywhere.
- High-Quality Imaging at Low Dose: It ensures both diagnostic accuracy and radiation safety, making it ideal for sensitive or mobile setups.
- Workflow-Friendly: Touchscreen controls, DICOM compatibility, and rapid setup make it ideal for urgent care and ergonomically demanding situations.
- Versatile Applications: Perfect for home healthcare, remote screening, bedside diagnostics, and emergency deployments.
The PRORAD Atlas Ultraportable X‑Ray System is the perfect blend of portability, performance, and ease-of-use. With a microprocessor-controlled high-frequency generator, ergonomic design, low radiation dose, and seamless digital workflows, it's ideally suited for modern medical scenarios that demand mobility and reliability.
PRORAD ATLAS ULTRAPORTABLE digital x-ray system is a state-of-the-art ultra-portable solution that comprises of high frequency x-ray generator that is microprocessor-controlled for precise x-ray delivery, an ergonomically and aesthetically designed unit with an LCD touch screen display of x-ray parameters and APR settings. It is a compact and lightweight X-ray imaging device that is designed for use in a variety of settings, including emergency rooms, intensive care units, and remote or mobile clinical settings.
TBSend Card
The TB Send Card, developed by Wobble Base in Surat, India, is an innovative, electricity-free solution aimed at enhancing the storage, safety, and transportation of diagnostic samples, particularly for tuberculosis (TB). This user-friendly device is engineered to store DNA for over six years, with easy retrieval by dipping the card into a one-step DNA release buffer, ready for direct use in CB-NAAT platforms such as GeneXpert. A short centrifugation step (2–5 minutes) can enhance DNA purity, and the card also supports magnetic bead–based purification and whole genome amplification through a 10-minute isothermal incubation, improving diagnostic sensitivity.
The card’s cellulose matrix is specially protected to prevent fungal growth, contamination, and DNA degradation, allowing long-term storage in simple laboratory or field conditions without refrigeration.
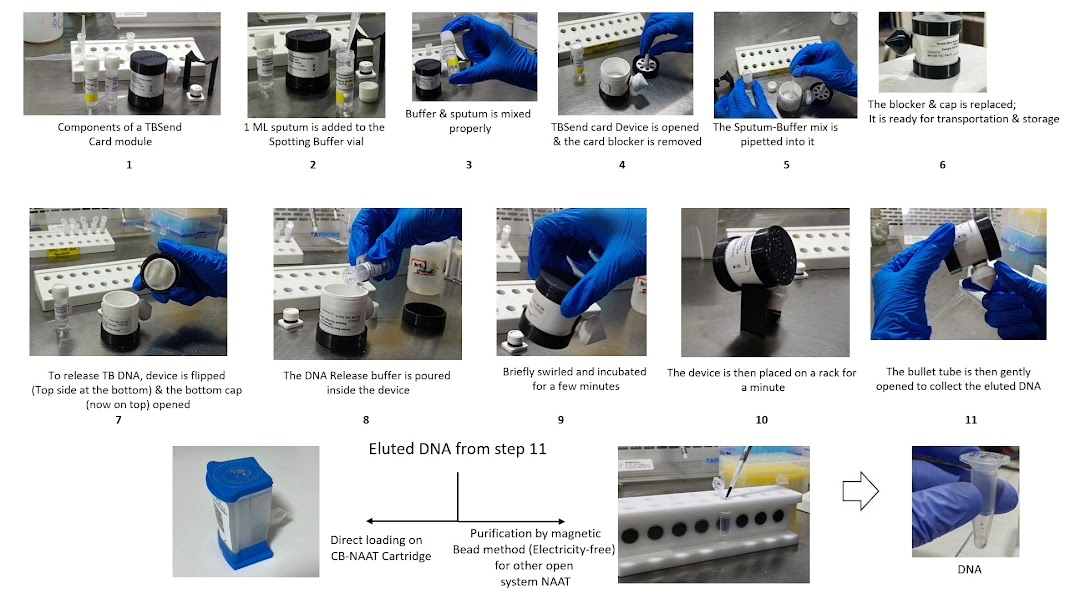
How TBSend Card works
Sputum is collected from patients and mixed with a provided spotting buffer and then applied in parts onto the cellulose card inside the device. The card is secured with a blocker and closed with a screw cap to allow drying, after which it can be safely transported and stored at room temperature without refrigeration. At the laboratory, the card is reopened, the blocker discarded, and a small punch of the dried sputum spot is taken for DNA release and further molecular testing (e.g., CB-NAAT, Xpert, or LPA). This system ensures biosafety, eliminates the need for a cold chain, prevents contamination, and provides stable DNA suitable for multiple TB diagnostic platforms.
Collect sputum → spot on card → dry → store/ship at room temp → punch card in lab → extract DNA → run Xpert/LPA/WGS.
Rationale and advantages
- Sample loss during transportation: Currently, ~10% of sputum samples get contaminated in transit under conventional systems. TB Send Card aims to eliminate that.
- Expected reductions: Up to ~70% reduction in loss of samples due to absence of cold chain.
- Increase in mean DNA concentration: Approximately ~25% higher diagnostic yield compared to conventional storage methods.
- Significant cost savings: Per-test cost is substantially lower than existing technologies.
- Eliminates or drastically reduces cold-chain dependency, a major bottleneck in remote or resource-limited settings.
- Enables safe sputum transport and storage at room temperature, removing the need for refrigeration and simplifying logistics.
- Reduces sample loss and degradation, mitigating the risk of contamination common in conventional transport.
- Cost-effectiveness: The annual report estimates a cost of around INR 5 per test (≈ US$0.06) for the card and INR 25 (≈ US$0.30) including extraction—significantly cheaper than alternatives costing >INR 165 (≈ US$2).
- Long-term stability: Samples can be safely stored from 1 day to over 6 years without loss of diagnostic quality.
- Patented innovation: The device is protected by both process and design patents, representing the first-of-its-kind integrated system for TB sputum collection, transport, and DNA release in an electricity-free format.
How TB Send Card Fits into the Diagnostic/TB Care Pathway
- In sample collection: After sputum is collected, instead of transporting raw samples or using a cold chain, the sample is spotted on the TB Send Card containing the spotting buffer and cellulose matrix.
- In transport: Room-temperature stability allows easy and low-cost transport to reference or central laboratories without refrigeration.
- In testing, DNA can be directly released for use in CB-NAAT (GeneXpert) or purified for other NAAT methods, including LPA and qPCR.
- In archival: Cards can serve as long-term “nucleic acid archives,” preserving genetic material for future retesting, epidemiological studies, or drug resistance analysis.
Development and Validation
The research and development of the TB Send Card was supported by the Bill & Melinda Gates Foundation under the Grand Challenges–Tuberculosis Control (GC-TBC) Phase 1 and Phase 2 programs.
The device cleared stringent biosafety assessment tests conducted at the ICMR–National Institute for Research in Tuberculosis (ICMR-NIRT), Chennai, funded by the India Health Fund (Tata Trusts) under the TBSend Card Phase 1 program. Following this, it has advanced to a national-level efficacy evaluation (Phase 2) at an ICMR institute, again with continued support from the India Health Fund (Tata Trusts).
The TB Send Card has demonstrated satisfactory efficacy, with results published in a peer-reviewed journal, and has obtained a test license from the Central Drugs Standard Control Organization (CDSCO)—a major milestone in its regulatory pathway in India.
Stage: Validated (functional prototype developed, biosafety cleared, CDSCO test license obtained, national evaluation ongoing).
- A functional prototype has been developed and validated.
- Biosafety evaluation completed at ICMR–NIRT, Chennai.
- Test license obtained from CDSCO, Government of India.
- Currently undergoing a national-level efficacy study funded by the India Health Fund (Tata Trusts).
ERAY SMART-5HS Portable Handheld X-Ray Machine
Compact. Intelligent. Game-Changing Point-of-Care Imaging Solution. The ERAY SMART 5HS Portable Handheld X-Ray Machine is a new-generation diagnostic imaging device designed for rapid, high-quality X-rays anywhere—clinics, rural health camps, ICUs, or TB mass-screening vans. Weighing just 3.9 kg, it combines Edusoft’s expertise in radiology with advanced AI-driven TB detection to deliver hospital-grade imaging at the patient’s doorstep.

The ERAY SMART 5HS represents the next step in portable radiology—a high-frequency, battery-operated handheld X-ray generator integrated with Edusoft’s proprietary ERAY Gold Premium DR Detector and ERAY ImageSuite AI Software. Together, these create a fully digital ecosystem capable of instant image acquisition, automatic TB screening, and wireless data transfer to hospital servers or cloud PACS—empowering faster and more reliable clinical decisions.
Key Features
- Ultra-Lightweight & Portable Design
- Weight: 3.5 – 3.9 kg only.
- Operated easily with one hand—ideal for mobile, ICU, or field setups.
- Effortless support-stand mounting for stability in constrained spaces.
- AI-Powered Tuberculosis Screening
- Integrated CXR TB AI trained on thousands of radiographs (CE & FDA cleared).
- Detects TB, pneumonia, and lung abnormalities in seconds with heat-map visualization.
- Enables on-the-spot triage and early disease detection in resource-limited areas.
- Reliable Battery-Driven Operation
- 22.2 V Li-Po battery (1450 mAh) supports up to 100 shots per charge / 6-8 hours of continuous use.
- Independent of mains power—perfect for rural or emergency deployments.
- High-Quality Digital Imaging
- Output range: 50–80 kV (1 kV step) | 2–5 mA (1 mA step).
- When paired with ERAY Gold DR, images appear within 3 seconds on the console/laptop.
- Delivers sharp, low-dose radiographs
- User-Friendly Control Interface
- 7″ LCD display (800×480) with dual-side operation buttons.
- Remote control (3 m range) minimizes radiation to the operator.
- Intuitive layout—no complex setup or calibration needed.
- Durable, Hygienic Design
- Flat, smooth housing allows quick sanitization.
- Resistant to dust and splashes—ideal for mobile clinics and rural environments.
ERAY Gold Premium DR Detector + ERAY ImageSuite Software
- Lightweight, hot-swap battery DR panel with 32 GB storage (> 1500 images).
- Fast Wi-Fi connectivity, auto-mode switching, and DICOM integration.
- AI Modules: TB AI, Bone Fracture AI, and Bone Suppression AI.
- 1.5× faster workflow, secure patient data, and advanced post-processing tools.
AI TB Software for Mass Screening
Through Edusoft’s partnership with VUNO Med-Chest X-ray (Korea, EU-CE & US FDA cleared), the ERAY SMART-5HS enables AI-assisted chest radiography for public health programs.
Feature | Benefit |
Automated AI detection | Instantly flags TB & pneumonia lesions |
Heat-map visualization | Identifies exact lung regions affected |
Abnormality score | Aids quick triage & radiologist confirmation |
Data integration | Exports to PACS |
Ideal for NTCP camps, NGOs, CSR initiatives, mobile vans, and rural screening centers.
Simplified Technical Specifications
Parameter | Specification |
Power Source | 22.2 V Li-Polymer Battery (1450 mAh) / 25.2 V DC Charger (100–240 VAC, 50–60 Hz) |
Output Range | 50–80 kV (1 kV step), 2–5 mA (1 mA step) |
Exposure Time | 0.03 – 2.00 s (Selectable steps 10 ms / 50 ms) |
Display | 7″ LCD (800 × 480 pixels resolution) |
X-Ray Tube | Stationary Tube with 0.8 mm Focal Spot |
Filtration | Inherent 0.5 mm Al + Additional 1.2 mm Al |
Dimensions/Weight | 266 (W) × 163 (H) × 260 (D) mm / ≈ 3.9 kg |
Battery Performance | Up to 100 exposures per charge / 1.5–2 hours continuous operation |
AI Integration | CXR TB Screening AI + Fracture AI + Bone Suppression AI modules (optional) |
Connectivity | Wireless Wi-Fi / LAN / DICOM Compatibility |
Data Storage | Linked to ERAY Gold DR Detector (32 GB Internal Storage / 1500+ Images) |
Certifications | AERB/BIS/NABL Certified/ISO 13485/IEC 60601/US FDA |
Clinical Validation | ICMR Validated for Chest X-Ray Imaging and TB Screening Applications |
Use Cases | TB Mass Screening, Mobile Health Units, ICU/NICU Critical Care, Veterinary Use, Rural Healthcare, and Disaster Response |
PathoDetect PCR Kits with Resistance Detection
The PathoDetect MTB RIF & INH Resistance Detection Kit is a robust, indigenous RT-PCR that offers fast, sensitive, and comprehensive TB diagnosis—including drug resistance profiling in under 2 hours. It consolidates tests into a single, streamlined assay. It's globally aligned yet tailored for India’s specific public health needs, with minimal infrastructure requirements and deep integration into national TB control systems..png)
Features of PathoDetect TB Kits
- Fully Integrated Sample-to-Result Workflow
- The PathoDetect MTB RIF & INH Resistance Detection Kit is designed for use with the Mylab Compact™ device, offering fully automated testing from sample loading to result generation in under 2 hours.
- This represents a closed NAAT system, where steps like extraction, amplification, and detection occur within a sealed, self-contained environment—minimizing manual intervention and contamination risk.
- Automated, High-Throughput Platform
- Supports parallel processing—handling multiple samples in one run (typically up to 16) and delivering robust contamination control, ease-of-use, and scalability.
- By combining the cartridge-based kit with an automated PCR platform, the workflow is streamlined and operator-independent, a hallmark of closed-system designs.
- Ambient-Friendly Design:
- Operates effectively without cold storage (unlike traditional cold-chain-dependent PCR kits), suited for rural, mobile, or low-infrastructure setups
- Multiple TB Testing Modalities
- MTB Detection Kit: Rapidly identifies Mycobacterium tuberculosis using multicopy sequence IS6110 for enhanced sensitivity.
- MTB/NTM Discrimination Kit: Differentiates MTB from non-tuberculous mycobacteria (NTM).
- MTB RIF & INH Resistance Detection Kit: Simultaneously detects TB and key drug-resistance markers for rifampicin and isoniazid.
- All kits emphasize high sensitivity, low contamination risk, quality control, and fast results as system hallmarks.
- Regulatory Approval & Field Validation
- The MTB RIF & INH kit is India’s first made-in-India TB detection kit with CDSCO, TB Expert Committee, and ICMR approvals and has gone through multicenter clinical evaluation, reinforcing reliability and performance.
The PathoDetect TB kits, especially the MTB RIF & INH Resistance Detection Kit, function as a closed NAAT system—offering a sealed, automated, and efficient workflow with rapid (approx. 2-hour) results and strong regulatory backing. These features facilitate deployment even in semi-automated or near-patient settings, supporting India’s TB elimination goals.
